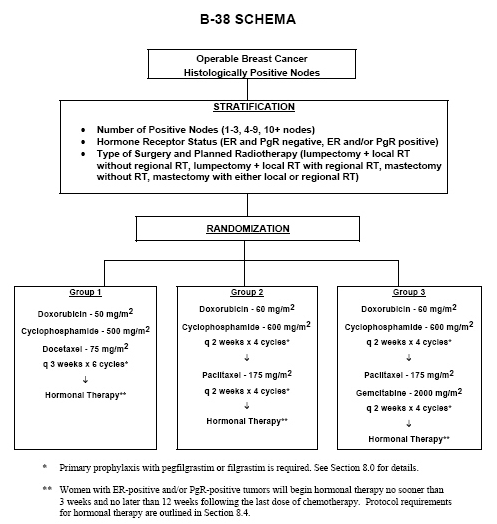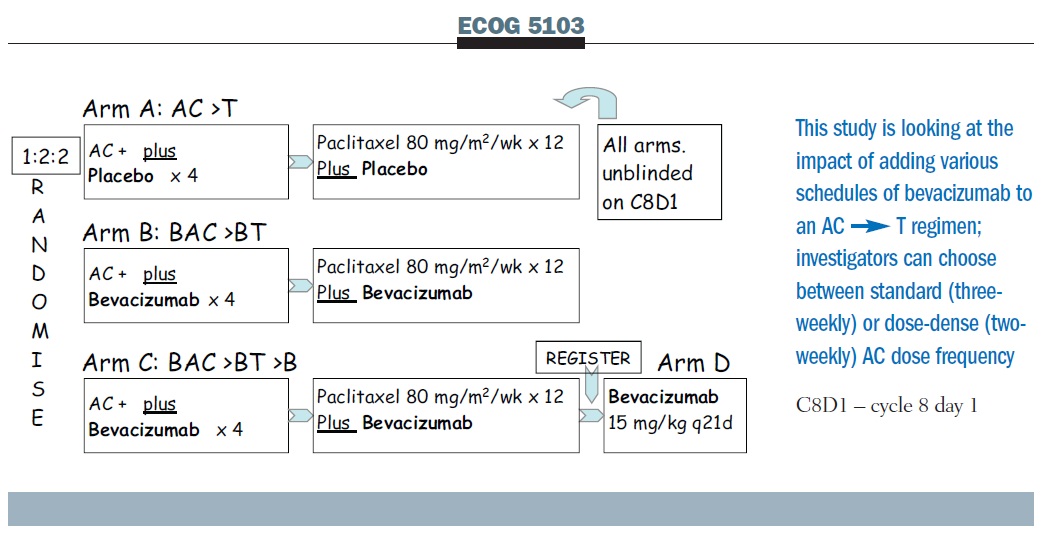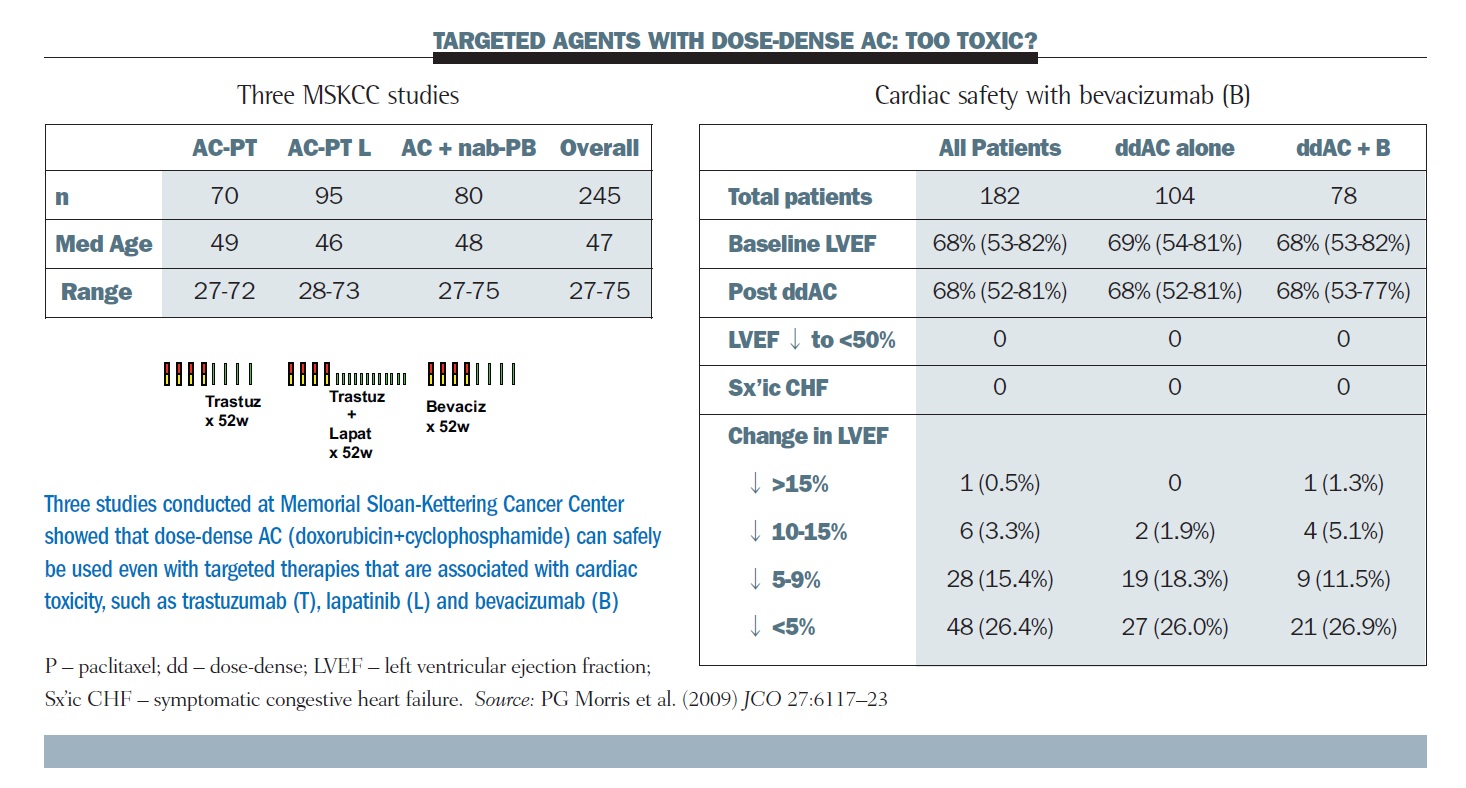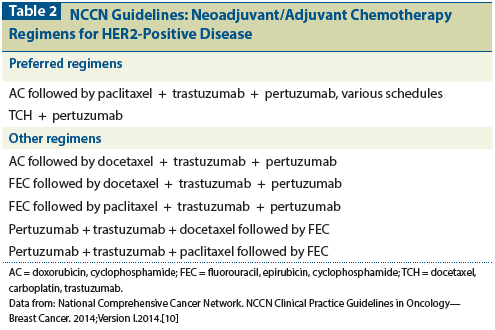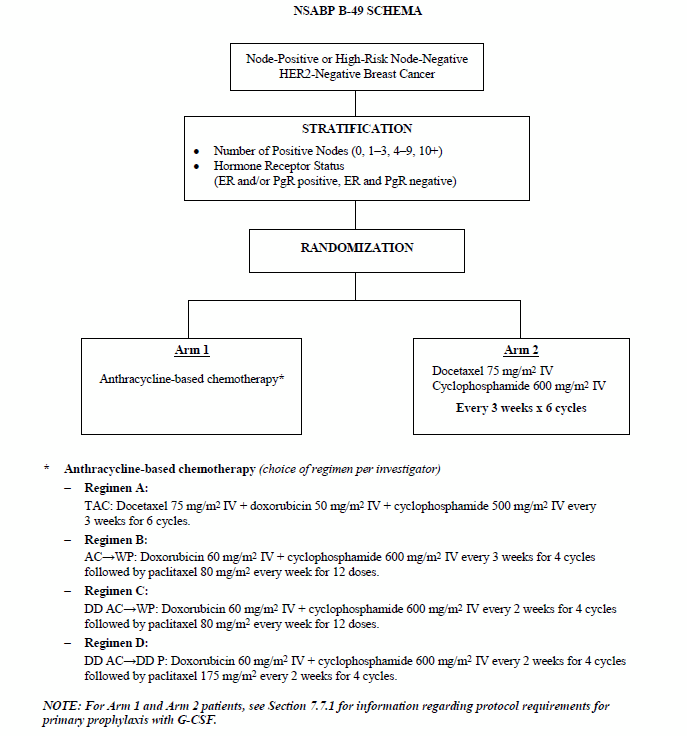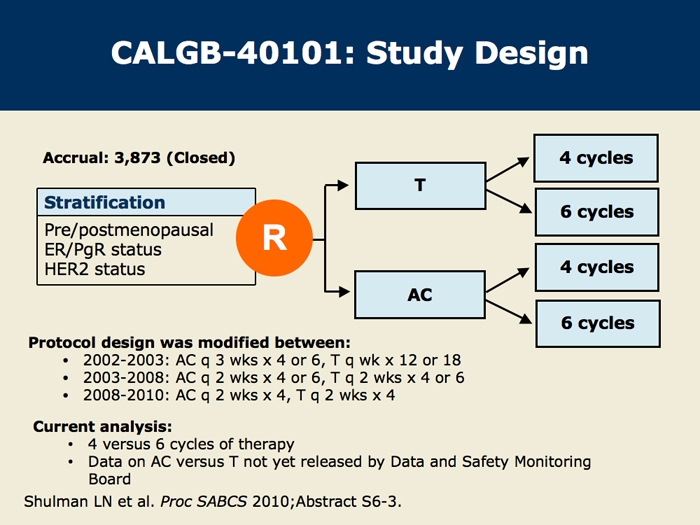
Effect of Number of Treatment Cycles of Adjuvant Chemotherapy on Clinical Outcomes | Research To Practice

Standardizing Chemotherapy Regimen Nomenclature: A Proposal and Evaluation of the HemOnc and National Cancer Institute Thesaurus Regimen Content | JCO Clinical Cancer Informatics
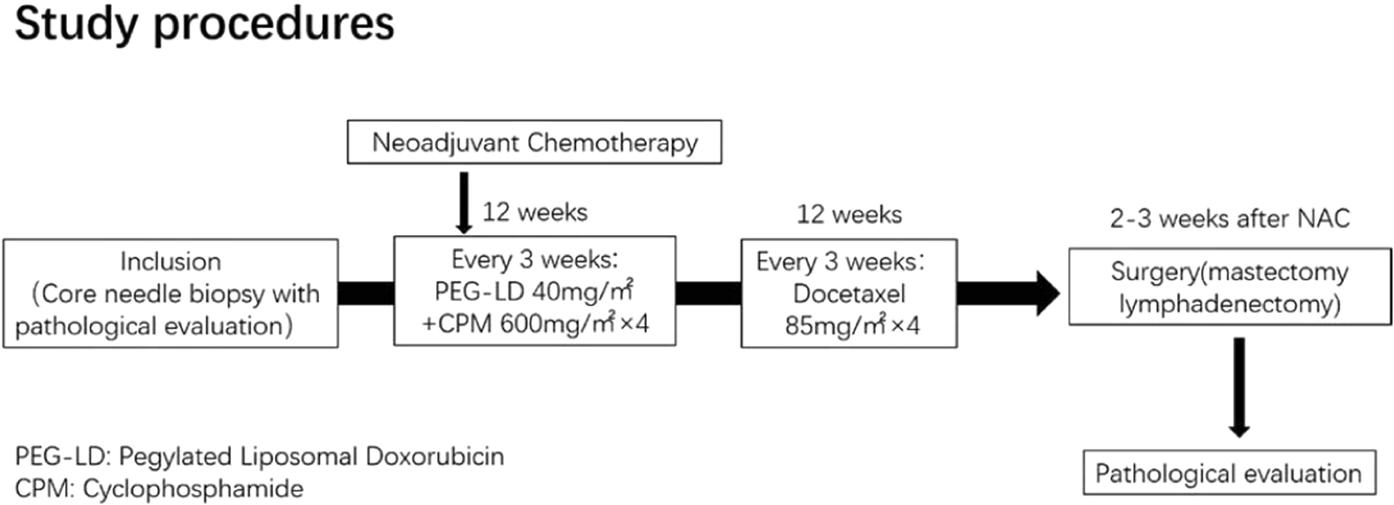
Pegylated liposomal doxorubicin plus cyclophosphamide followed by docetaxel as neoadjuvant chemotherapy in locally advanced breast cancer (registration number: ChiCTR1900023052) | Scientific Reports

Anthracycline-free or short-term regimen as adjuvant chemotherapy for operable breast cancer: A phase III randomized non-inferiority trial - The Lancet Regional Health – Western Pacific

PDF) Dose Dense Doxorubicin and Cyclophosphamide Followed by Paclitaxel (Dose Dense AC Followed by T) Regimen for Breast Cancer
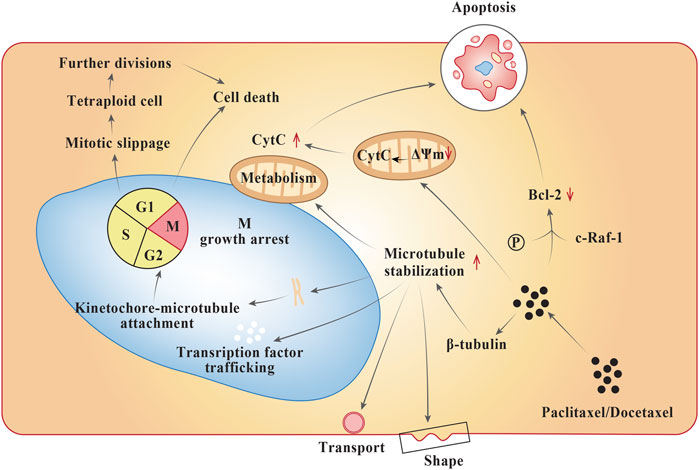
Frontiers | Platinum and Taxane Based Adjuvant and Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer: A Narrative Review
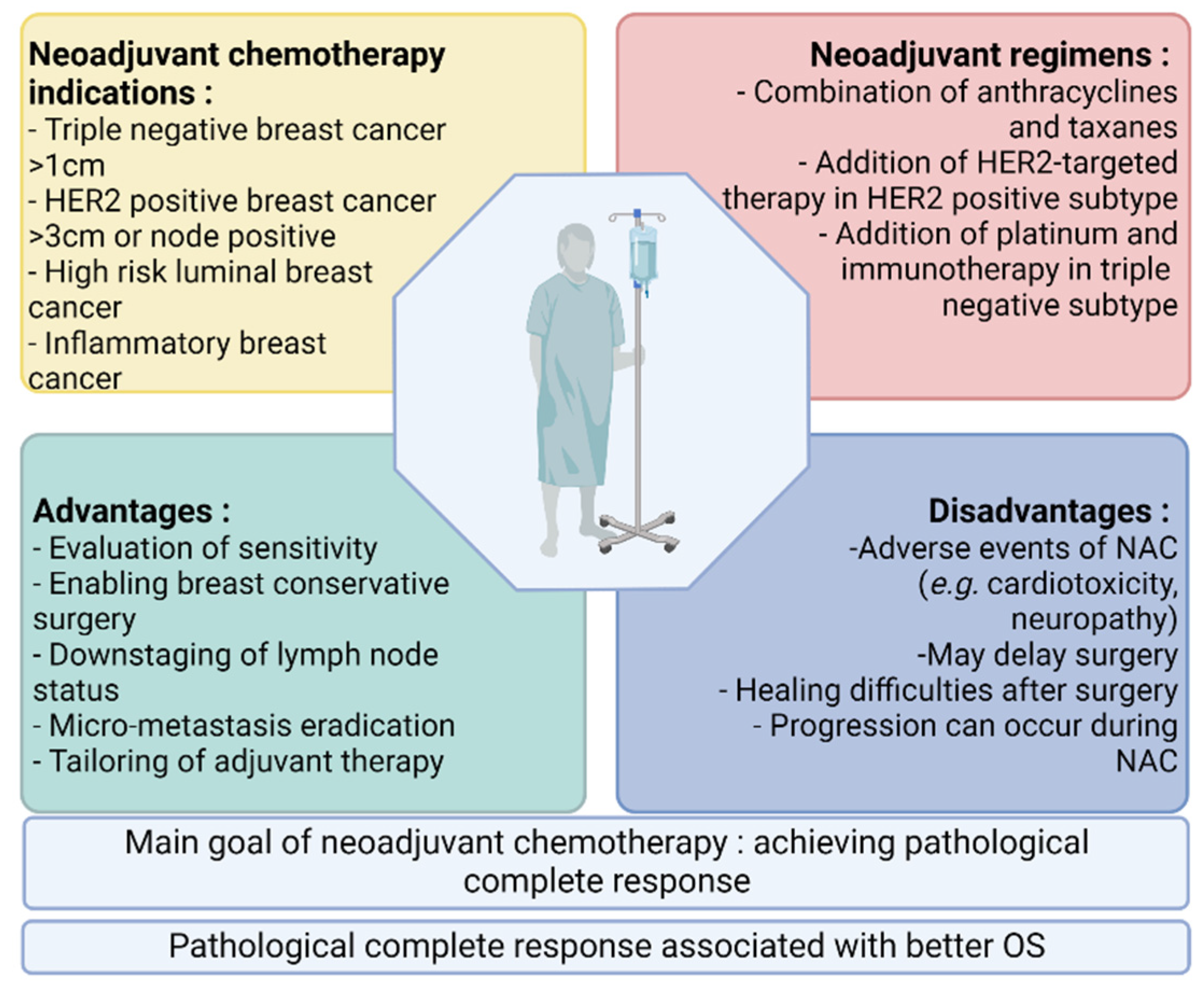
Cancers | Free Full-Text | Predictive Biomarkers of Response to Neoadjuvant Chemotherapy in Breast Cancer: Current and Future Perspectives for Precision Medicine

SWOG S 0800 ( NCI CDR 0000636131 ) : addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response ( pCR ) rates in inflammatory or locally
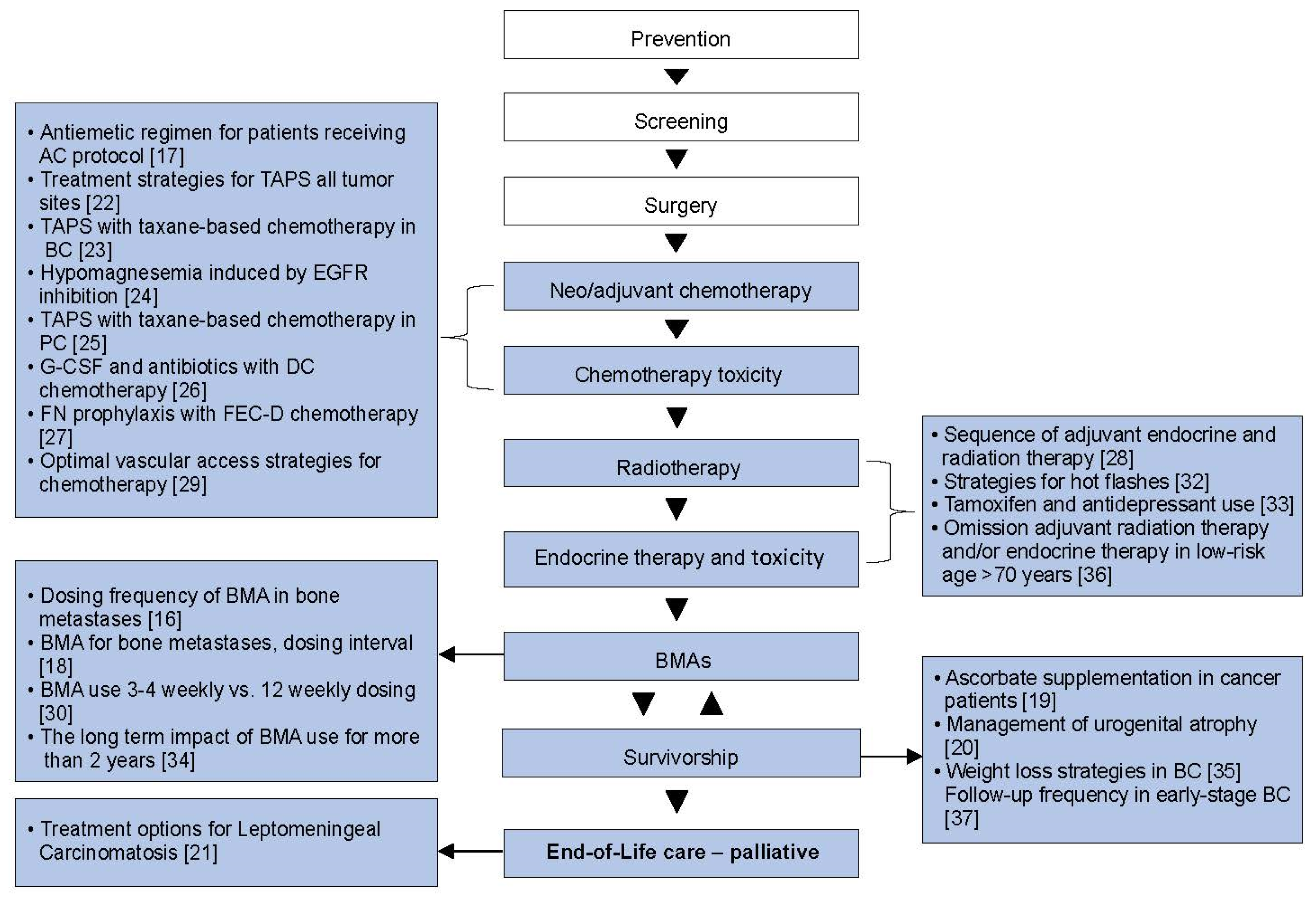
Current Oncology | Free Full-Text | Integrating Systematic Reviews into Supportive Care Trial Design: The Rethinking Clinical Trials (REaCT) Program
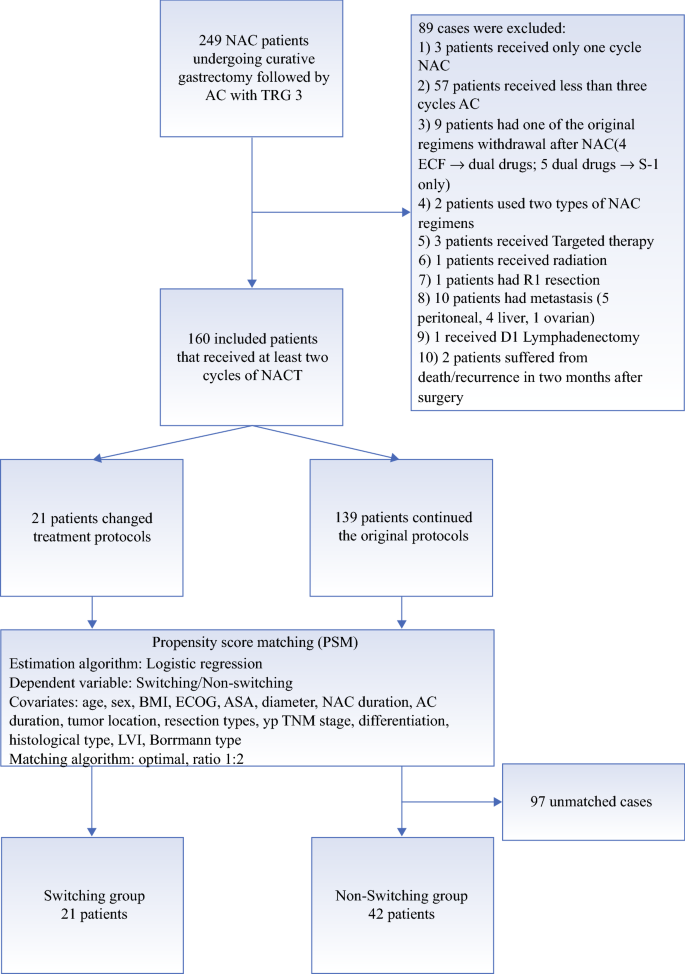
Treatment Switch in Poor Responders with Locally Advanced Gastric Cancer After Neoadjuvant Chemotherapy | SpringerLink